NewTn - SAFETY (Pharmaceutical Safety Management System Software)
-
Payment
WK
-
MOQ
Negotiable
-
Country of sale
World Wide
-
PRICE
-
EXW
Depend on quantity
-
ITEM SPECIFICS
-
Brand
NewTn
-
origin
Republic of Korea
-
Size(Capacity)
Web Application
PRODUCT DESCRIPTION
NewTn SAFETY Detailed Description
Overview
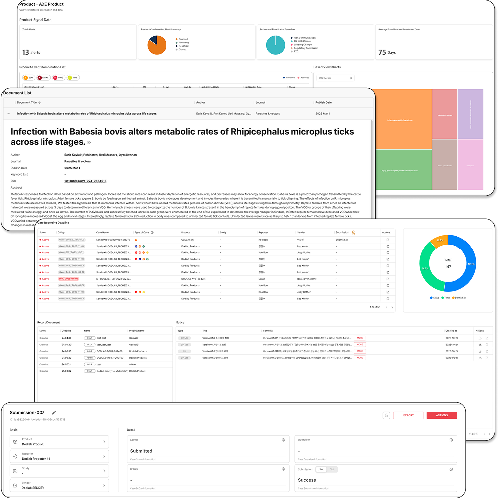
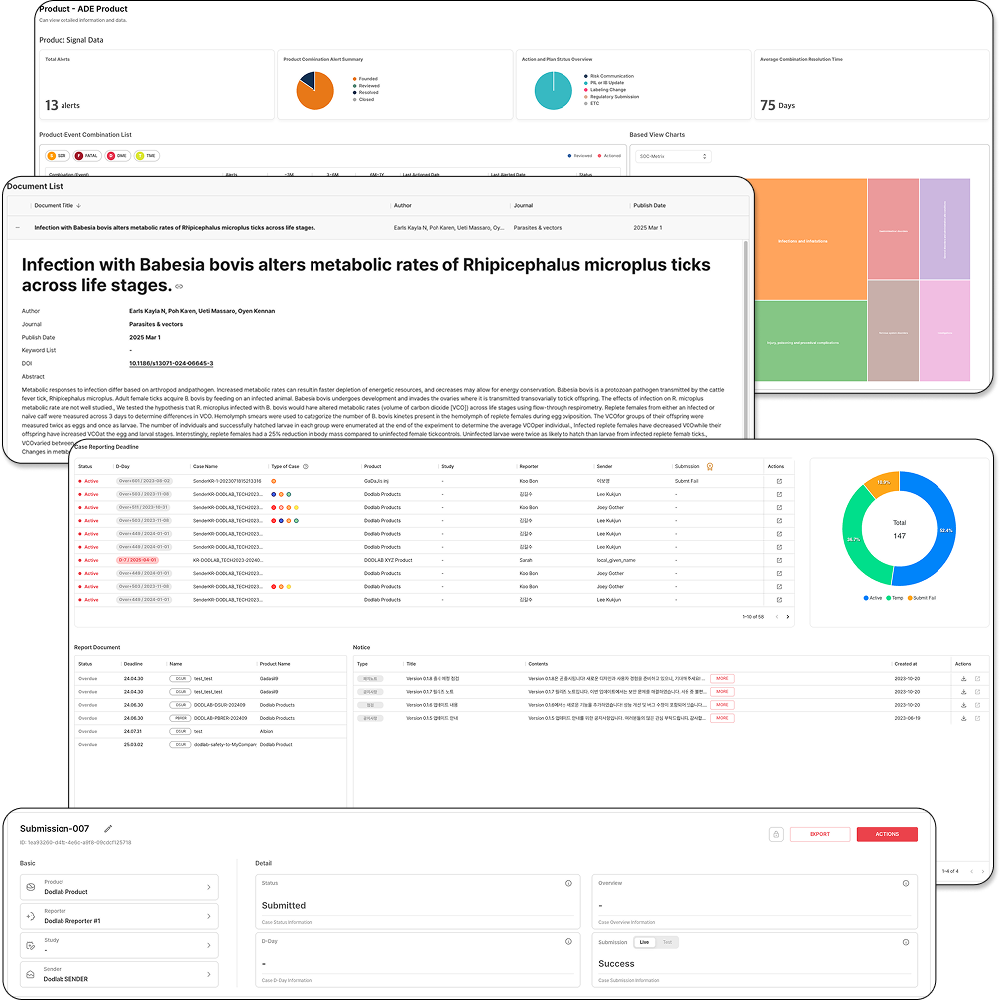
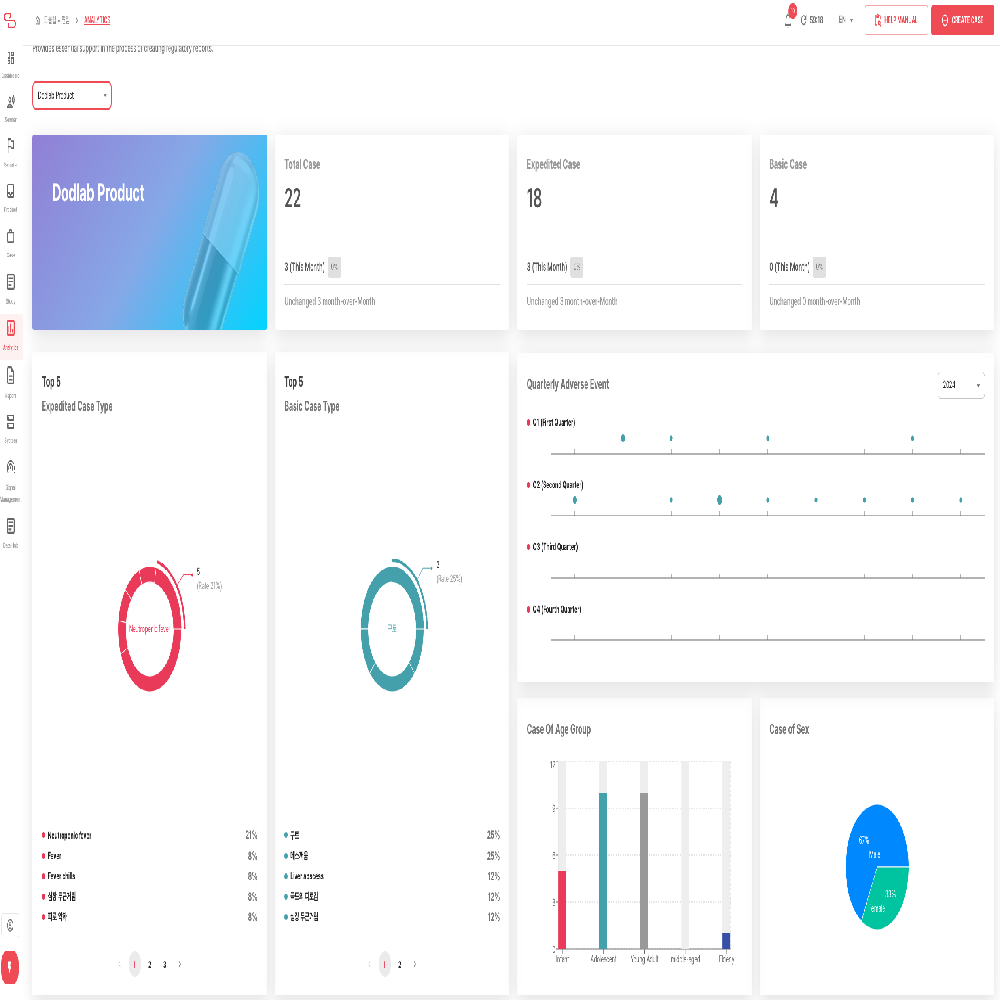
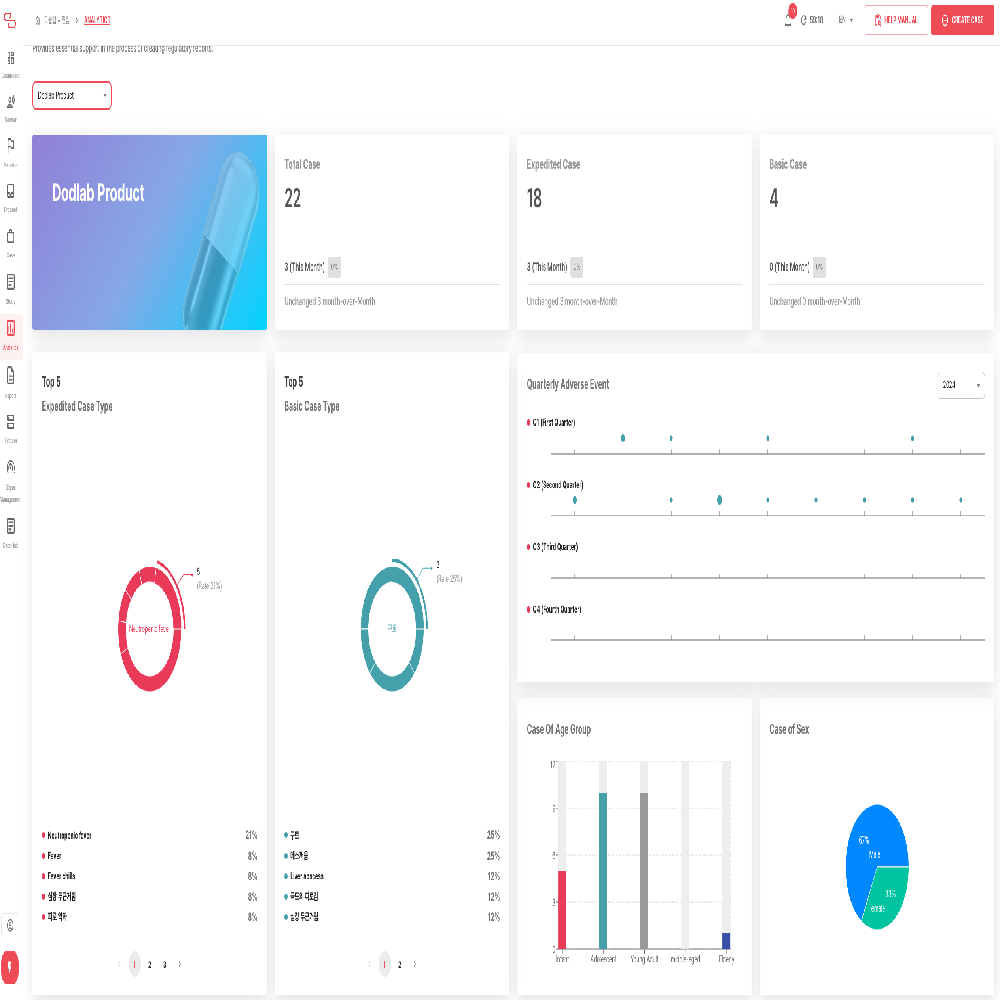
NewTn SAFETY is an integrated solution designed for comprehensive lifecycle safety management of pharmaceuticals and medical products. It supports efficient and systematic pharmacovigilance operations, meeting both domestic and international regulatory requirements. The system covers the entire process, from individual case reporting to periodic safety report preparation, literature monitoring, data analysis, and regulatory submissions, ensuring both user convenience and data accuracy.
Key Features
1. ICSR-Based Individual Case Management
• Preparation and management of Individual Case Safety Reports (ICSR) compliant with domestic and global regulatory standards
• Stepwise process management from case intake, assessment, coding, review, and submission
• Support for international coding systems such as MedDRA
2. Electronic Reporting and Regulatory Submission Support
• XML generation and submission functions for electronic reporting
• Compliance with E2B(R3) international standard format for global regulatory submissions
• Submission tracking and history management
3. Support for Periodic Safety Report Preparation
• Automatic generation and management of various periodic safety reports, including DSUR (Development Safety Update Report) and PBRER (Periodic Benefit-Risk Evaluation Report)
• Cumulative data analysis and risk assessment support
4. Literature Search and Safety Information Monitoring
• Provision of up-to-date academic literature search functionality
• Collection of safety information from search results and linkage with case reports
5. System Management and User Access Control
• Role-based user access control and audit trail management
• Enhanced data integrity and security features
6. User-Friendly Interface
• Intuitive screen design with step-by-step work guides
• Customizable interface and features based on user needs
Key Strengths
• Optimized system for compliance with domestic regulatory requirements
• Compliance with global pharmacovigilance standards and support for multi-country reporting
• Maximized data accuracy and report preparation efficiency
• Rapid response to regulatory changes with continuous updates and support
Target Users
• Pharmaceutical company pharmacovigilance teams
• Contract Research Organizations (CRO) and pharmacovigilance service providers
• Safety management departments of medical device and biotechnology companies
Conclusion
NewTn SAFETY is a comprehensive pharmacovigilance solution that enhances data accuracy and operational efficiency across the entire process of safety information collection, analysis, and reporting, while ensuring full compliance with both domestic and global regulatory requirements.
PAYMENTS DETAILS
- WK
- Name : Jihoon Lee
SHIPPING
- 161-8 Magokjungang-ro, Gangseo-gu, Seoul (07788)
The person in charge
Jihoon LeeAddress
161-8 Magokjungang-ro, Gangseo-gu, Seoul (07788)
dodlab qr
-
- Business Type :
- Knowledge Service
-
- Main Product :
- NewTn - SAFETY : Pharmacovigilance Management System
-
- Established :
- 2023-11-01
-
- Total Annual Revenue :
- 3~5 million (KRW)
-
- Total Employees :
- Less than 5
Please suggest a variety of your ideas such as design, impact, enhancements, etc
Please enter the text on the left image to prevent automatic input.
0 / 4000
CUSTOMER REVIEWS (0)
TRADE EXPERIENCE
-
- Total revenue
- 3~5 million (KRW)
-
- Total export revenue (previous year in USD)
-
- Number of foreign trade employees
- Less than 5
COMPARISON TO SIMILAR ITEMS more
- No Items
- supplier level
- MEMBER
- Dodlab, Inc.
- Seller's Store url
- Response Level
★ ★ ★ ★ ★

- Supplier Level
★ ★ ★ ★ ★

- Transaction Level
★ ★ ★ ★ ★

- No Items